Abstract
Introduction: Operations with medicines require compliance with the requirements established in Good Practices with the objective of guaranteeing their quality, safety and effectiveness. The Quality Management System incorporates quality risk management as an integral part. In the injectable plant, the aseptic processing of Cephalosporins and Carbapenems is carried out in the form of sterile powders for injection. Production is subject to special requirements to minimize the risks of microbial, particulate and pyrogen contamination. The environmental microbiological monitoring program is one of the critical elements in the production process; it must be applied routinely and periodically. The objective of this work is to carry out risk assessment in the environmental monitoring process of controlled areas of the plant by identifying the risk points and the causes that originate them and establishing preventive and/or corrective measures to minimize their frequency and impact on processes. Materials and Methods: Failure Modal Analysis and Process Effects are used for risk assessment; it is a prevention method aimed at achieving Quality assurance. Supporting techniques such as Brainstorming and Cause-Effect Diagram were also used. Results: Through the application of this technique, reference criteria are obtained for monitoring viable and non-viable particles in the environment, monitoring frequencies, sampling frequency and number of sampling points according to m2 of the area to be monitored and determination of points by priority level. Discussion: A flow chart of the process is built to analyze the inputs of raw materials and the most critical points where contamination can be generated for different causes. The identification of risks was carried out by applying the brainstorming technique and as a result, 4 critical areas with a high probability of contamination occurrence were determined. By building the Ishikawa diagram of the process to be improved, the environmental monitoring program becomes a powerful tool to avoid the undesired effect, which is product rejection. The risk priority number was calculated for each failure mode and corrective measures were established to mitigate the effect level. Conclusions: As a result of the risk assessment in the environmental monitoring process of controlled areas, risks were identified and evaluated in order of criticality. Preventive and/or corrective measures were established for each stage in order to reduce the possibility of risks of product contamination.
|
Published in
|
Pharmaceutical Science and Technology (Volume 8, Issue 2)
|
|
DOI
|
10.11648/j.pst.20240802.13
|
|
Page(s)
|
47-55 |
|
Creative Commons
|

This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited.
|
|
Copyright
|
Copyright © The Author(s), 2024. Published by Science Publishing Group
|
Keywords
Risk Assessment, Environmental Monitoring, Injectables
1. Introduction
Operations with medicines require compliance with the requirements established in Good Manufacturing Practices (GMP), with the aim of guaranteeing their quality, safety and effectiveness and thus ensuring the interests of the patient, society and the state
.
The GMP are an essential part of the Quality Management System (QMS), which incorporates Quality Risk Management (ARC) as an integral part. GMP and ARC are two fundamental requirements that must be aligned in an integrated quality system
.
In November 2005, the Steering Committee of the International Conference on Harmonization, ICH, signed the ICH Q9 Quality Risk Management (QRM) guideline in Chicago. Since that moment, the term "Risk Management" has infiltrated all areas of pharmaceutical production. In 2019, the guide was updated with a new definition of risk as the uncertainty that affects the objectives set by a company. Therefore, every day it is more evident that risk management is a necessary component of an effective QMS, by allowing the assessment, control, communication and review of the quality risks of a product throughout its life cycle
. In this way, defects can be prevented, safety and consumer satisfaction increased.
Risk management is a requirement for all establishments dedicated to the manufacturing, storage, commercial distribution and/or import of medicines for human or research use, as well as for quality control laboratories, in such a way that it constitutes a a quality risk management system closely related to the quality assurance system
| [6] | Mora, Roman. Ortiz, Angie. 2021. Risk analysis in the pharmaceutical industry: Development of a standardized operating procedure in a pharmaceutical company in Costa Rica. Research R&D Magazine Vol 16.n2 Jul-Dec 2021. https://doi.org/10.33304/revinv.v16n2-2021008 |
[6]
.
In the case of the pharmaceutical sector, knowing the risks they face or may face is even more necessary as these are industries that constitute a highly competitive market, require very long-term investments and with high needs for research and development programs I+D+I
.
The manufacture of sterile products is subject to special requirements to minimize the risks of microbial, particulate and pyrogen contamination. They are carried out in clean areas, with access through locks for personnel, raw materials and materials.
Clean areas maintain an adequate level of cleanliness and must be provided with air filtered through high-efficiency HEPA filters. They are classified according to the quality of the environment expressed in humidity, temperature and pressure and the amount of particles in the air under rest and operating conditions. They are microbiologically monitored and the entrances and exits to them are controlled
.
The environmental microbiological monitoring program is one of the critical elements in the production process, it must be applied routinely and periodically and the data must be obtained by adequately trained technicians using controlled instruments and culture media prepared and approved in accordance with current quality control standards. Each process must be carefully evaluated when selecting sampling sites with the objective of providing data that is useful to help identify current or potential contamination problems associated with specific procedures, equipment, materials and processes. Those places that, if contaminated, would contaminate the product should be sampled. However, it may be prudent to select sites close to but not in contact with the product.
.
The importance of this program is that it allows demonstrating the effectiveness of the sterilization processes, as well as identifying that the products are being manufactured under conditions where the facilities, equipment, personnel and processes involved comply with those established in the Good Manufacturing Practices. Manufacturing, which allows products to have the required quality and level of asepsis, where potential contaminants are minimized or eliminated and potential contamination routes can be quickly identified that allow the respective corrections to be implemented in a timely manner. Since the release of products without adequate sterile conditions may cause them to be withdrawn from the market, and in the worst case scenario, cause serious implications for public health and even put the lives of patients at risk
.
The Injectable Cephalosporins and Carbapenems Plant belonging to the "8 de Marzo" Pharmaceutical Company is dedicated to the dosage and packaging of antibiotics in the pharmaceutical form of powders for injections. It has an installed production capacity of 14 million vials per year. The facility has separate areas for washing and depyrogenizing bulbs, moist heat sterilization of caps, aluminum seals, technological clothing and format pieces; dosing and caking in aseptic area, visual inspection, labeling and packaging, they also have the equipment and premises necessary to carry out the operations carried out.
As a medicine producing company, its QMS is certified by ISO 9001:2015, and it has introduced risk management as part of its business strategy with the need to remain a sustainable company over time and market a successful quality product. The production of injectables is mainly intended for therapy rooms in hospitals, caring for seriously ill patients.
The main objective of the regulations governing GMP is to reduce the risks inherent in all pharmaceutical production that cannot be completely prevented by definitive control of the products. Essentially, such risks are of two types:
1. Cross contamination
2. Confusion
One of the most used tools for risk analysis is Failure Modal and Effects Analysis (FMEA). It was developed as a formal design methodology in the 1960s by the aerospace industry, and was later used by the mechanical and automotive industries, such as Ford in the United States
.
It is a logical, systematic and progressive technique to identify and eliminate potential and/or known failures and/or errors in any design, process or system. Therefore, it has been introduced as a valid technique for risk assessment
.
The central aspect of an FMEA is the calculation of the Risk Priority Number (RPN), the combination of the severity, probability and sometimes detectability of a failure mode. Based on the NPR, risks can be prioritized and subsequently mitigated or eliminated.
.
FMEA is a method used to evaluate failures that may occur in a process and their probable consequences on the results and behavior of the products. It is based on knowledge of the product and the process, it is a powerful tool to identify the most important failures that can occur, the factors causing these failures and their possible repercussions. FMEA results can be used as a basis for design
. It is the risk analysis methodology that has been most frequently used in the pharmaceutical industry.
The general objective of this work is to apply FMEA as a tool for risk assessment in the environmental monitoring process of controlled areas of the plant based on:
1. Identify risk points and the causes that originate them.
2. Establish preventive and/or corrective measures to minimize their frequency and impact on processes.
3. Obtain the sampling points and monitoring frequency of the most critical ones.
2. Materials and Methods
Risk assessment can be used to focus control strategy development on only those aspects with significant potential to impact quality. The ICH Q9 definition of risk assessment mentions the combination of the probability of harm occurring and the severity of that harm, risk assessment as the identification of hazards, analysis and evaluation of the risks associated with the exposure to those hazards
.
The fundamental questions related to risk assessment are the following:
1. What can go wrong?
2. What is the probability that something will go wrong?
3. What are the consequences?
To make risk-based decisions, a systematic approach is essential. The ICH Q9 guideline, Quality Risk Management, provides a structure for initiating and following a risk management process.
The pharmaceutical industry and regulators can also assess and manage risks using recognized risk management tools or internal procedures (e.g. standard operating procedures). Some of the simple techniques commonly used to structure risk management by organizing data, facilitating decision making, are: flowcharts, checklists, process diagrams, cause/effect diagrams (also called Ishikawa or fishbone diagrams)
.
Failure Mode Analysis and its Effects is a prevention method aimed at achieving quality assurance, which through a systematic analysis allows us to evaluate, from the design phase of a product, service or process, the probability of occurrence of failures. a failure, its severity and the possibility of its detection.
In this case we will use the process FMEA where the product failures derived from possible process failures are analyzed until its delivery to the customer.
The FMEA methodology follows the following steps:
.
1. Select the group of experts: for this, 5 Specialists with extensive experience in the Company and knowledge about injectable production were selected. The Principal Microbiology Laboratory Specialist was designated as the leader of the activity.
2. Establish the type of FMEA to be carried out, its purpose and limits: in this case it is a process, a flow chart was built with the following objectives:
a. Define the process, which includes the parameters for each unit operation.
b. Identify the product journey and its most critical steps.
c. Evaluation and implementation of process mechanisms, where the interaction and function of man as the main executor of the process and its main contaminant is understood, in addition to the product, equipment, facilities and the environment.
d. Review of the context of quality issues for each process: review of quality deviations, out of analytical specifications, control of changes in process instructions, review of product quality specifications.
3. Determine the Potential Failure Modes for each stage of the process: this identification is a critical step; another alternative technique is used, known as Brainstorming logical deduction processes based on experience and knowledge of the process. The experts know the activities of their processes and have experience, a simple methodology is to hold meetings in which the members express their knowledge through group work and begin to collect information for subsequent classification.
4. Determine Potential Failure Effects: Each failure mode can have several potential effects.
5. Determination of Potential Causes of Failure, whether direct or indirect: for this case, another tool known as the Cause and Effect Diagram was used.
Figure 2. Cause-Effect Diagram.
6. Identify current control systems: controls designed to prevent possible causes of failure, both direct and indirect, or to detect the resulting failure mode are sought.
a. The different events that may constitute risks were identified to comply with the objectives of the process: once the risks are identified, they are evaluated. The values corresponding to the Severity (S), Occurrence (O) and Detectability (D) of the failures were assigned through teamwork, to obtain the Risk Priority Number (RPN), it is a value that allows prioritize failure modes and their causes, which were identified in the FMEA analysis:
b. Severity (G): Potential impact of a failure mode on the production process. The greater the possible severity, the greater their number would be and the lower the severity, the lower their number would be.
c. Probability (P): This parameter is related to the fact that the probabilities increase to the extent that the events have happened in the past. For events that have never occurred, the probability, without being zero, is considered low. When a new production line is started, it is common for low values to be assigned to this parameter, since lack of experience means that the risks have not materialized.
d. Detection (D): Refers to the possibility of detecting events that occur in relation to the detected risks. If they are not detectable, then the value assigned is high These parameters must be organized and structured by levels, where depending on the scale used, they must indicate the degree to which each one affects the process. Generally the scale has 10 levels, which allows for better precision, but 5 levels are also used, which will depend on how the organization wishes to evaluate them.
Table 1. Reference criteria.
Level | Severity | Occurrence | Detectability |
1 | Minimal impact | Does not occur | Null detection |
2 | Indirect impact, does not compromise the quality of the product. | Unlikely | Low detection |
3 | Moderate impact | Moderate probability | Moderate detection |
4 | High impact. | Affects quality Likely to pass or repeat | High probability of detection |
5 | Very serious impact. Causes rejection of the product. | Very high probability. Happens constantly. | Very high probability of detecting the fault. |
The NPR is obtained by multiplying the levels of Severity (G), Probability (P) and Detactibility (D) obtained by mode.
The higher the NPR value, the higher the priority of the risk and the more urgent preventive or corrective actions will be required to minimize its frequency and impact on the processes, for which an action plan is established for each proposed failure mode.
Taking into account that the contamination failure of the product is the most critical and that it impacts the quality and can reach the product rejection processes, the team of experts as a result of the Cause-effect diagram focused on the monitoring process environmental as a critical element of the production process, by establishing the corrective actions for each failure mode according to the value of the highest NPR, the sampling points and the monitoring frequency of the most critical ones are determined.
3. Results
Identification of risk points and the causes that originate them.
The technological processes of the plant were analyzed, establishing as critical areas:
1. Aseptic area.
2. Materials entry area.
3. Sterilization and filtration area.
4. Bulb washing area.
These are areas of contamination risk for the product in the production process where raw materials and primary materials are mostly exposed to cross contamination. In addition to being clean areas classified as class A/B, C and D according to the ISO 14644-1 standard.
Taking these criteria into account, the expert group applies the FMEA method for the Environmental Monitoring activity. From the point of view of microbiological control of the facilities, people, equipment and environment, this activity guarantees that the processes are carried out within the established quality parameters and conditions and with the minimum of risks.
A table is made for each area identified as a potential risk, the different failure modes are identified (how and what can fail?). The effects of the failure (What is its impact when it occurs?), in this case the Severity is evaluated. The causes of the failure (What can cause the failure mode?), in this case the Occurrence is evaluated. The controls to be established are identified for all causes of failure, and the detectability number is determined. With these three values, the NPR is calculated for each failure mode.
Table 2. Extract from the FMEA application table.
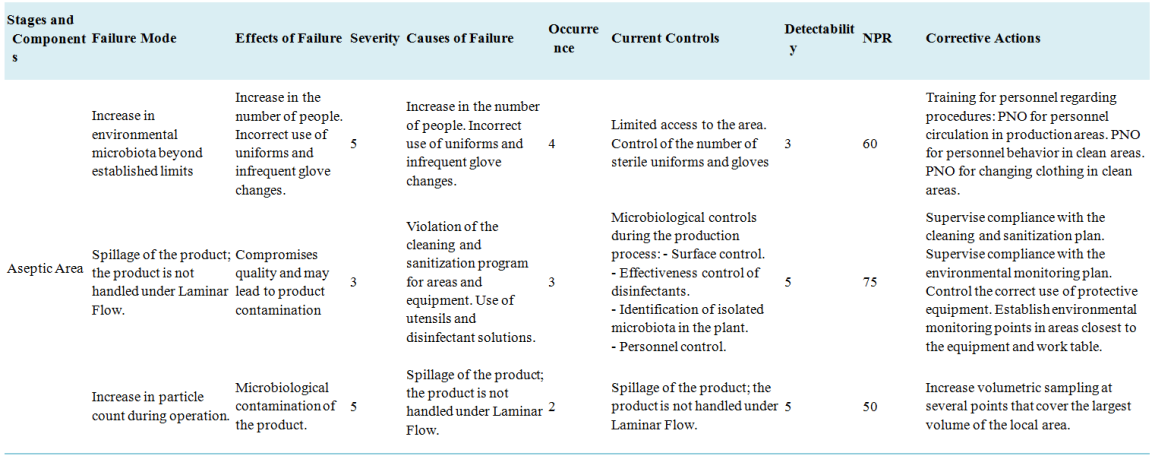
Own elaboration
Each expert makes these assessments which are then reconciled and grouped in descending order by the lead expert based on the NPR.
As a result of this evaluation, the failure modes with the highest NPR are determined:
1. Traces of product in the environment and on equipment surfaces.
2. Increase in environmental microbiota.
3. High possibility of contamination from man to product.
4. Detection of traces of products in the equipment.
5. Increased particle count in operation.
Taking these aspects into account, the reference criteria are determined:
1. Monitoring of non-viable particles in the environment.
Table 3.
2. Monitoring of viable particles in ambient air.
Table 4.
3. Monitoring of viable particles on contact surfaces.
Table 5.
4. Personnel Monitoring.
Table 6.
Table 3. Monitoring of Non-Viable Particles in the Environment.
Sampling rate | Sampling Locations by Local |
No. of points | Location of points by priority |
Daily and/or batch produced. Recommended continuous measurement. | Depending on the size of the premises | Close to operation and/or handling areas at a distance of 30 - 100 cm. Close to operation and/or handling areas at a distance of more than 100 cm. In areas with higher personnel traffic and movement of materials and equipment. In areas accessed by personnel and material transfer. |
Daily and/or batch produced in more critical locations. At least once a week in less critical locations. |
At least once a week |
At least once a month |
Table 4. Monitoring of viable particles in ambient air.
Sampling rate | Sampling Locations by Local |
No. of points | Location of points by priority |
Daily and/or per batch produced. | Based on the characteristics of the facility and the operations carried out within it. | Volumetric sampling at one or more points that cover the largest volume of air in the facility. Plates exposed at ± 100 cm from critical operation and/or handling areas as a supplement if necessary. |
Between 2 – 3 times a week in higher criticality locations. At least once a week in lower criticality locations. |
At least once a week |
At least once a month |
Table 5. Monitoring of Viable Particles on Contact Surfaces.
Sampling rate | Sampling Locations by Local |
No. of points | Location of points by priority |
Daily and/or per batch produced. | Based on the characteristics of the facility and the operations carried out within it. | Contact plates on surfaces linked to operation and/or handling areas and swabbing on hard-to-reach surfaces, preferably at the end of the workday. Contact plates or, if not available, swabbing of surfaces in areas with higher personnel traffic and material transfer, during or at the end of the workday. Contact plates or, if not available, swabbing of surfaces in areas accessed by personnel and material transfer. |
Between 2 – 3 times a week in higher criticality locations. At least once a week in lower criticality locations. |
At least once a week |
At least once a month |
Table 6. Personnel monitoring.
Sampling rate | Sampling Locations by Local |
No. of points | Location of points by priority |
Daily and/or per batch produced. | All those who perform high-impact operations. | Digital impressions of both hands on contact plates, preferably at the end of the workday. Impressions using contact plates on clothing (sleeves, collar, and chest) during the established qualification periods. |
At least once a week. | Not applicable. |
Not applicable. | All those who perform high-impact operations. |
Depending on the areas determined to be the most critical and taking into account the results of the risk analysis. Environmental monitoring points are determined. For example, in the aseptic area the points are: Oven outlet (Laminar Flow), Bulb turntable, Dosing equipment, Feeding hopper, Capping equipment, Autoclave outlet (Laminar flow), Aseptic area entrance airlock, Anti-vibration table of the analytical balance.
4. Discussion
The first action of the group of experts was to build the process flowchart and from there analyze the inputs of raw materials and the most critical points where contamination can be generated due to different causes during the process. The identification of risks was carried out through the application of the brainstorming technique and as a result, 4 critical areas were determined during the process, with a high probability of contamination occurring. These clean areas where the product transits, for example: Area classified as D, vial washing machine where the bulbs are exposed, the operator must carry out this operation according to GMP using gloves that do not shed particles and be properly dressed in a uniform that does not shed particles. All this behavior is important to reduce the level of contamination towards the bulb that is in direct contact with the product.
By building the Ishikawa diagram on the most critical failure or the process to be improved, the environmental monitoring program becomes the most powerful tool to prevent the occurrence of the undesired effect that is product rejection. With the application of FMEA, the risk priority number (RPN) was calculated for each failure mode and corrective measures were established to mitigate the effect level.
The evaluation of risks related to critical processes during the aseptic manufacturing of injectables is regulatory, the GMP requires using techniques that allow you to determine the most vulnerable stages where the medication can be contaminated and from there take preventive or corrective actions to mitigate the risks. since its occurrence is a probabilistic phenomenon.
This study is based on other risk assessments of the environmental monitoring process in other laboratories in the biopharmaceutical industry, which carry out aseptic operations. In the particular case of this study, it is specific because we are the only company in the country dedicated to aseptic dosing of Sterile powders in pharmaceutical form of injectables.
5. Conclusions
As a result of the risk assessment in the environmental monitoring process of controlled areas, risks were identified and evaluated in order of criticality. Preventive and/or corrective measures were established for each stage in order to reduce the possibility of risks of product contamination. In addition, this study allowed us to know the usual microbiota in the facility, investigate the sources of contamination, evaluate the effectiveness of cleaning and disinfection procedures, methods and agents, as well as recovery methods.
Abbreviations
GMP | Good Manufacturing Practices |
QMS | Quality Management System |
ARC | Quality Risk Management |
ICH | International Conference on Harmonization |
FMEA | Failure Modal and Effects Analysis |
NPR | Risk Priority Number |
Conflicts of Interest
The authors declare no conflicts of interest.
References
| [1] |
Velasco, Claudia. 2018. QRM - Quality Risk Management in the Pharmaceutical Industry. Available at:
https://cercal.cl/envinculo/qrm/
|
| [2] |
CECMED, 2012. Risk Management Guide. Available at:
https://www.cecmed.cu/sites/default/files/adjuntos/Reglamentacion/Guia%20adm%20de%20riesgos.pdf
|
| [3] |
Rodriguez, S. 2020. Webinario Gestión de Riesgos. Conceptos y aplicaciones en la industria farmacéutica. Disponible en:
https://www.akrimet.com
|
| [4] |
ICH Q9. (2005). Harmonised Tripartite Guideline. Quality Risk Management, Q9. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. European Union, Japan and USA. Available in:
https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirementsregistration-pharmaceuticals-human-use-ich-guideline-q9-quality-risk-management-step-5-first-version_en.pdf
|
| [5] |
Urciuolo, Andrea. 2024. Quality Risk Management and ICH Q9 in the Pharmaceutical Industry. Available at:
https://blog.pqegroup.com/es-es/cumplimiento-gxp/gestion-de-riesgos-de-calidad-ich-q9-en-la-industria-farmaceutica
|
| [6] |
Mora, Roman. Ortiz, Angie. 2021. Risk analysis in the pharmaceutical industry: Development of a standardized operating procedure in a pharmaceutical company in Costa Rica. Research R&D Magazine Vol 16.n2 Jul-Dec 2021.
https://doi.org/10.33304/revinv.v16n2-2021008
|
| [7] |
Anton, Ana P. 2024. Importance of risks in the pharmaceutical industry. Available at:
https://blog.Softexpert.com
|
| [8] |
ASMONTEC, 2022. What is a Clean Room in the Pharmaceutical Industry? Published article. Available at:
https://asmontec.com.br/es/area-limpa-na-industria-farmaceutica/
|
| [9] |
Airedinámica, 2021. Clean Rooms in the Pharmaceutical Industry. Available at:
https://www.airedinamica.com/cuartos-limpios/salas-blancas-en-la-industria-farmaceutica-y-sus-mejores-practicas/
|
| [10] |
Avila, M. 2021. El proceso de monitoreo ambiental en las áreas limpias en la industria farmacéutica y su automatización. Available at:
https://www.linkedin.com/pulse/el-proceso-de-monitoreo-ambiental-en-las-%C3%A1reas-limpias-marcela-avila/
|
| [11] |
WIKIPEDIA. 2023. Failure Mode and Effects Analysis, 2024. Available at:
https://es.wikipedia.org/wiki/An%C3%A1lisis_modal_de_fallos_y_efectos
|
| [12] |
Jhuéz, J. (2018). Metodologías para gestión del riesgo. España: Universidad Politécnica de Catalunya. Recuperado el 26 de septiembre de 2018,
https://capacitacioncgr.jovenclub.cu/wp-content/uploads/2018/05/Metodologia-para-la-Gestion-del-Riesgo.pdf
|
| [13] |
McDermott, R. E, Mikulak. (2008). The Basics of FMEA (2 ed). Productivity Press book.
https://doi.org/10.1201/b16656
|
| [14] |
Ministry of Labor and Social Affairs of Spain (MITES), 2004: NTP 679: Failure Mode and Effects Analysis (FMEA). Available at:
https://www.insst.es/documents/94886/326775/ntp_679.pdf/3f2a81e3-531c-4daa-bfc2-2abd3aaba4ba
|
| [15] |
Guía para el desarrollo del análisis de modo y efecto de falla 2017 | primera edición. Available at:
http://cufcd.edu.mx/calidad/v20/documentacion/CM/CEMA-MN-CA-2.pdf
|
| [16] |
Sphera’s Editorial Team, June 21, 2022. What is Failure Mode and Effects Analysis (FMEA) Available at:
https://sphera.com>glosario-es
|
| [17] |
INFINITIA Industrial Consulting. What is FMEA: Failure Mode and Effects Analysis. April 21, 2021. Available at:
https://www.infinitiaresearch.com
|
Cite This Article
-
APA Style
Sagre, X. M., Martínez, S. G., Aldama, N. O. (2024). Risk Assessment for Environmental Monitoring of the Injectable Cephalosporins and Carbapenems Production Plant. Pharmaceutical Science and Technology, 8(2), 47-55. https://doi.org/10.11648/j.pst.20240802.13
 Copy
|
Copy
|
 Download
Download
ACS Style
Sagre, X. M.; Martínez, S. G.; Aldama, N. O. Risk Assessment for Environmental Monitoring of the Injectable Cephalosporins and Carbapenems Production Plant. Pharm. Sci. Technol. 2024, 8(2), 47-55. doi: 10.11648/j.pst.20240802.13
 Copy
|
Copy
|
 Download
Download
AMA Style
Sagre XM, Martínez SG, Aldama NO. Risk Assessment for Environmental Monitoring of the Injectable Cephalosporins and Carbapenems Production Plant. Pharm Sci Technol. 2024;8(2):47-55. doi: 10.11648/j.pst.20240802.13
 Copy
|
Copy
|
 Download
Download
-
@article{10.11648/j.pst.20240802.13,
author = {Xenia Madrazo Sagre and Susana Gómez Martínez and Nancy Oña Aldama},
title = {Risk Assessment for Environmental Monitoring of the Injectable Cephalosporins and Carbapenems Production Plant
},
journal = {Pharmaceutical Science and Technology},
volume = {8},
number = {2},
pages = {47-55},
doi = {10.11648/j.pst.20240802.13},
url = {https://doi.org/10.11648/j.pst.20240802.13},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.pst.20240802.13},
abstract = {Introduction: Operations with medicines require compliance with the requirements established in Good Practices with the objective of guaranteeing their quality, safety and effectiveness. The Quality Management System incorporates quality risk management as an integral part. In the injectable plant, the aseptic processing of Cephalosporins and Carbapenems is carried out in the form of sterile powders for injection. Production is subject to special requirements to minimize the risks of microbial, particulate and pyrogen contamination. The environmental microbiological monitoring program is one of the critical elements in the production process; it must be applied routinely and periodically. The objective of this work is to carry out risk assessment in the environmental monitoring process of controlled areas of the plant by identifying the risk points and the causes that originate them and establishing preventive and/or corrective measures to minimize their frequency and impact on processes. Materials and Methods: Failure Modal Analysis and Process Effects are used for risk assessment; it is a prevention method aimed at achieving Quality assurance. Supporting techniques such as Brainstorming and Cause-Effect Diagram were also used. Results: Through the application of this technique, reference criteria are obtained for monitoring viable and non-viable particles in the environment, monitoring frequencies, sampling frequency and number of sampling points according to m2 of the area to be monitored and determination of points by priority level. Discussion: A flow chart of the process is built to analyze the inputs of raw materials and the most critical points where contamination can be generated for different causes. The identification of risks was carried out by applying the brainstorming technique and as a result, 4 critical areas with a high probability of contamination occurrence were determined. By building the Ishikawa diagram of the process to be improved, the environmental monitoring program becomes a powerful tool to avoid the undesired effect, which is product rejection. The risk priority number was calculated for each failure mode and corrective measures were established to mitigate the effect level. Conclusions: As a result of the risk assessment in the environmental monitoring process of controlled areas, risks were identified and evaluated in order of criticality. Preventive and/or corrective measures were established for each stage in order to reduce the possibility of risks of product contamination.
},
year = {2024}
}
 Copy
|
Copy
|
 Download
Download
-
TY - JOUR
T1 - Risk Assessment for Environmental Monitoring of the Injectable Cephalosporins and Carbapenems Production Plant
AU - Xenia Madrazo Sagre
AU - Susana Gómez Martínez
AU - Nancy Oña Aldama
Y1 - 2024/10/31
PY - 2024
N1 - https://doi.org/10.11648/j.pst.20240802.13
DO - 10.11648/j.pst.20240802.13
T2 - Pharmaceutical Science and Technology
JF - Pharmaceutical Science and Technology
JO - Pharmaceutical Science and Technology
SP - 47
EP - 55
PB - Science Publishing Group
SN - 2640-4540
UR - https://doi.org/10.11648/j.pst.20240802.13
AB - Introduction: Operations with medicines require compliance with the requirements established in Good Practices with the objective of guaranteeing their quality, safety and effectiveness. The Quality Management System incorporates quality risk management as an integral part. In the injectable plant, the aseptic processing of Cephalosporins and Carbapenems is carried out in the form of sterile powders for injection. Production is subject to special requirements to minimize the risks of microbial, particulate and pyrogen contamination. The environmental microbiological monitoring program is one of the critical elements in the production process; it must be applied routinely and periodically. The objective of this work is to carry out risk assessment in the environmental monitoring process of controlled areas of the plant by identifying the risk points and the causes that originate them and establishing preventive and/or corrective measures to minimize their frequency and impact on processes. Materials and Methods: Failure Modal Analysis and Process Effects are used for risk assessment; it is a prevention method aimed at achieving Quality assurance. Supporting techniques such as Brainstorming and Cause-Effect Diagram were also used. Results: Through the application of this technique, reference criteria are obtained for monitoring viable and non-viable particles in the environment, monitoring frequencies, sampling frequency and number of sampling points according to m2 of the area to be monitored and determination of points by priority level. Discussion: A flow chart of the process is built to analyze the inputs of raw materials and the most critical points where contamination can be generated for different causes. The identification of risks was carried out by applying the brainstorming technique and as a result, 4 critical areas with a high probability of contamination occurrence were determined. By building the Ishikawa diagram of the process to be improved, the environmental monitoring program becomes a powerful tool to avoid the undesired effect, which is product rejection. The risk priority number was calculated for each failure mode and corrective measures were established to mitigate the effect level. Conclusions: As a result of the risk assessment in the environmental monitoring process of controlled areas, risks were identified and evaluated in order of criticality. Preventive and/or corrective measures were established for each stage in order to reduce the possibility of risks of product contamination.
VL - 8
IS - 2
ER -
 Copy
|
Copy
|
 Download
Download
